As well as the development of databases directed toward the analysis of insect samples. In agreement with uniform practice in biology, we analyzed three replicates of fecal samples per triatomine species. This sample size does not allow variance stabilization, which usually occurs when n>100. Therefore, some metabolites that were considered part of the uniform core may actually belong to the variable core. In contrast, metabolites from the variable core are unlikely to belong to the uniform core, 4-(Benzyloxy)phenol simply because if they can be considered statistically variable with only three repetitions, the level of certainty can only increase with repetition number. In fact, statistical confidence in the determination of whether a metabolite is part of the uniform or variable core can be obtained with three replicates because the threshold associated with this choice is based on a much larger sample size of metabolites, which warrants statistical consistency according to p#0.05. Limitations notwithstanding, we used DI-FT-ICR-MS to characterize the fecal metabolome of three species of triatomines and to identify subsets of metabolites that are either uniform to all species or variable among them. In doing so, we found that the metabolites conserved among the three species pertained to multiple metabolic classes, with fatty acids, steroids, glycerolipids, amino acids, sugars, and nucleotides being widely represented. As previously discussed, given that parasite differentiation takes place in the triatomine gut, the chemical environment encountered by T. cruzi is likely to affect this process. As such, the molecules described here as uniform to all species of triatomines may play key roles in the life cycle of the parasite. We showed that lipids and fatty acids are the most abundant metabolite classes in the feces of all triatomines studied. Lipids play a fundamental role in the biological cycle of T. cruzi. Lipid extracts from metacyclogenic Folinic acid calcium salt pentahydrate intestinal preparations were shown to induce significant differentiation of epimastigotes into infective metacyclic trypomastigotes. The authors also showed that the total fraction of blood lipids represented by lysophosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, phosphatidylcholine, triacylglycerol and sphingomyelin is quickly degraded into free fatty acids in the triatomine intestinal tract and is incorporated into epimastigotes. It is known that free fatty acids are imported into the trypanosomatid cell via an ABC transporter, a superfamily of ATP-binding cassette transporters. In addition, the rate of metacyclogenesis induction by the total lipid fraction was shown to be about half of that obtained 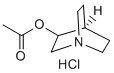 by a total intestinal extract and similar to that of free fatty acids or oleic acid alone. Thus, oleic acid alone is able to mimic half the rate of metacyclogenesis induced by the whole intestinal extract and promote trypomastigote viability and integrity. Another collateral effect observed by these authors was the biosynthesis of phosphatidylcholine and diacylglycerol by epimastigotes, as well as the activation of protein kinase C, as a consequence of free fatty acid accumulation due to the digestion of blood phospholipids by an intestinal phospholipase. This is an expected consequence of the inositol phosphate/diacylglycerol signaling pathway that has been described in T. cruzi. In this sense, the triatomine digestive process could be linked to metacyclogenesis via protein kinase C activation through diacylglycerol biosynthesis. The feedback regulation of diacylglycerol biosynthesis occurred through the correlated synthesis of phosphatidylcholine. Interestingly, the signaling pathway of metacyclogenesis induced by free fatty acids is different from that induced by cAMP.
by a total intestinal extract and similar to that of free fatty acids or oleic acid alone. Thus, oleic acid alone is able to mimic half the rate of metacyclogenesis induced by the whole intestinal extract and promote trypomastigote viability and integrity. Another collateral effect observed by these authors was the biosynthesis of phosphatidylcholine and diacylglycerol by epimastigotes, as well as the activation of protein kinase C, as a consequence of free fatty acid accumulation due to the digestion of blood phospholipids by an intestinal phospholipase. This is an expected consequence of the inositol phosphate/diacylglycerol signaling pathway that has been described in T. cruzi. In this sense, the triatomine digestive process could be linked to metacyclogenesis via protein kinase C activation through diacylglycerol biosynthesis. The feedback regulation of diacylglycerol biosynthesis occurred through the correlated synthesis of phosphatidylcholine. Interestingly, the signaling pathway of metacyclogenesis induced by free fatty acids is different from that induced by cAMP.