Hence, there is a need for identifying new antigens from L. donovani ideally relying on correlates of protective immunity. On the basis of the fact that recovery from VL is always associated with immunity to subsequent infection and induction of Th1 cytokines dominated by IFN-c, we identified several Th1 stimulatory proteins from soluble fraction and sub-fraction of L. donovani ranging from 89.9 to 97.1 kDa through proteomics which was also found to be protective against experimental VL. Triose phosphate isomerase of L. donovani, a vital glycolytic enzyme, was one such Th1 stimulatory Orbifloxacin protein identified from the above stated fraction of soluble Leishmania antigen. In this study, we have re-assessed LdTPI for its possible immunogenic and prophylactic potential against VL. LdTPI, which was cloned, expressed and purified, has the homology with L. infantum TPI to the tune of 99%. Immunoblot study of L.donovani promastigote lysate with the polyclonal anti-rLdTPI antibody revealed one dominant 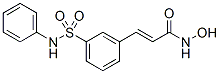 protein of,27 kDa. The Benzethonium Chloride presence of this protein in higher molecular weight range in proteomic studies, in contrast to its observed molecular mass could be attributed to the post-translational modifications which are widely prevalent in Leishmania. When evaluated for its immunogenicity by LTT and cytokine responses in PBMCs from cured/endemic/infected kala-azar patients, we observed,2.0 to 4.0 times better proliferative response as well as IFN-a? and IL-12 in comparison to SLD and low concentration of IL-10 in culture supernatants of rLdTPI induced PBMCs from cured kala-azar patients as well as endemic contacts. It is likely that the individuals from the endemic area are mostly exposed thus exhibiting such levels of responses. As expected, rLdTPI did not induce proliferation or cytokine production in healthy subjects, suggesting its specificity toward L. donovani infection. The analysis of cellular immune response of the rLdTPI was further validated in hamsters’ lymphocytes/ macrophages in order to correlate the observations made with the human PBMCs as the systemic infection of the hamster with L. donovani is very similar to human kala-azar. In the absence of cytokine reagents against hamsters, we have evaluated the effect of rLdTPI on LTT and NO production by peritoneal macrophages of hamster. It is well documented that in case of leishmanial infections, macrophages become activated by IFN-c released from parasite-specific T cells, and are able to destroy intracellular parasites through the production of several mediators, principal among which is NO. rLdTPI gave significantly higher cellular responses viz. LTT as well as NO release against all the cured hamsters in comparison to normal and infected ones. The limitations of this in vitro study based on a convenience human sampling, may not perhaps allow drawing solid conclusions regarding the immunogenicity of rLdTPI. However, since the cellular responses of the antigen in human subjects were further validated in cured hamsters, our findings therefore accentuate that L. donovani-primed PBMCs from cured kala-azar patients and hamsters after stimulation with rLdTPI exhibit a strong Th1-type cellular response, which should be protective in nature. Successful immunization that induces protection against leishmaniasis is highly dependent on adjuvants or delivery system that preferentially stimulates the Th1 phenotype of immune response and plasmid DNA is one of the most interesting vaccine delivery system. In contrast to conventional immunization that results in stimulating primarily CD4-T-cell responses, DNA immunization has been shown to stimulate both CD4- and CD8-T-cell responses. Based on this, in the present study, we carried out DNA vaccination with LdTPI- DNA in hamsters and challenged with the virulent strain of L. donovani.
protein of,27 kDa. The Benzethonium Chloride presence of this protein in higher molecular weight range in proteomic studies, in contrast to its observed molecular mass could be attributed to the post-translational modifications which are widely prevalent in Leishmania. When evaluated for its immunogenicity by LTT and cytokine responses in PBMCs from cured/endemic/infected kala-azar patients, we observed,2.0 to 4.0 times better proliferative response as well as IFN-a? and IL-12 in comparison to SLD and low concentration of IL-10 in culture supernatants of rLdTPI induced PBMCs from cured kala-azar patients as well as endemic contacts. It is likely that the individuals from the endemic area are mostly exposed thus exhibiting such levels of responses. As expected, rLdTPI did not induce proliferation or cytokine production in healthy subjects, suggesting its specificity toward L. donovani infection. The analysis of cellular immune response of the rLdTPI was further validated in hamsters’ lymphocytes/ macrophages in order to correlate the observations made with the human PBMCs as the systemic infection of the hamster with L. donovani is very similar to human kala-azar. In the absence of cytokine reagents against hamsters, we have evaluated the effect of rLdTPI on LTT and NO production by peritoneal macrophages of hamster. It is well documented that in case of leishmanial infections, macrophages become activated by IFN-c released from parasite-specific T cells, and are able to destroy intracellular parasites through the production of several mediators, principal among which is NO. rLdTPI gave significantly higher cellular responses viz. LTT as well as NO release against all the cured hamsters in comparison to normal and infected ones. The limitations of this in vitro study based on a convenience human sampling, may not perhaps allow drawing solid conclusions regarding the immunogenicity of rLdTPI. However, since the cellular responses of the antigen in human subjects were further validated in cured hamsters, our findings therefore accentuate that L. donovani-primed PBMCs from cured kala-azar patients and hamsters after stimulation with rLdTPI exhibit a strong Th1-type cellular response, which should be protective in nature. Successful immunization that induces protection against leishmaniasis is highly dependent on adjuvants or delivery system that preferentially stimulates the Th1 phenotype of immune response and plasmid DNA is one of the most interesting vaccine delivery system. In contrast to conventional immunization that results in stimulating primarily CD4-T-cell responses, DNA immunization has been shown to stimulate both CD4- and CD8-T-cell responses. Based on this, in the present study, we carried out DNA vaccination with LdTPI- DNA in hamsters and challenged with the virulent strain of L. donovani.