TAC was therefore chosen as a target pharmacophore for the preparation of analogues. Having observed that the simple modification of the parent TAC scaffold to create SRI-224 led to a greater than two-fold increase in potency, we investigated the effect of further simple structural modifications. To achieve this task we prepared a modest library of 31 compounds using a standard protocol for the generation of imines via the reaction of thiosemicarbazide, a derivative thereof, and a range of monosubstituted aromatic aldehydes. Furthermore, a more thorough comparative analysis of the mycolic acid profile resolved by two-dimensional silver nitrate-impregnated TLC plates showed that the inhibition of mycolic acid synthesis was accompanied by 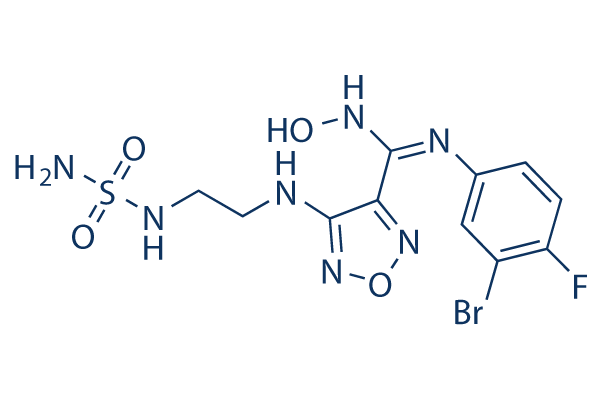 a modest, but reproducible, accumulation of unsaturated mycolic acids, presumably due to the inhibition of SAM-dependent methyltransferases involved in introducing cyclopropane rings on the meromycolates, as reported previously in other mycobacterial strains. While this effect was very limited following TAC LOUREIRIN-B treatment, accumulation of unsaturated keto-mycolic acid precursors was more pronounced after exposure to higher concentrations of 15 and 16, consistent with our previous observations using the SRI-224 analogue. One would expect that all mycolic acid subspecies would be inhibited concomitantly, leading to complete cessation in mycolic acid biosynthesis, as is seen with specific FAS-II inhibitors such as INH or ETH. FAS-II components are all essential and known to participate in the meromycolyl-ACP elongation steps, and it is during this process that the specific methyltransferases involved in the formation of the meromycolic acid are likely to operate. That keto-mycolic acids are refractory to TAC inhibition is somehow intriguing and argues against the HadABC being directly targeted by TAC. Supporting this view, both the recent study by Belardinelli and Morbidoni and the present study failed to identify mutations within the HadB subunit bearing the catalytic activity. Mutations only occurred in the HadA and HadC subunits, perhaps 4-(Benzyloxy)phenol stabilizing the complex and presenting the growing acyl-ACP substrate to HadB, thought to be the enzymatically active component of the complex. Elegant work demonstrated that the FAS-II system of M. tuberculosis is organized in specialized interconnected complexes composed of the condensing enzymes, dehydratase heterodimers and the methyltransferases. This led the authors to propose that because the interactions amongst these enzymes are crucial and their disruption detrimental for M. tuberculosis survival, the protein interactions could represent attractive drug targets. Whether this is the case for TAC remains to be established experimentally but seems highly conceivable. Our results also emphasize the possibility that EthA-activated TAC and related analogues are likely to inhibit mycolic acid biosynthesis by physically altering the interactions of HadAB and HadBC dehydratases with other FASII components, particularly those involved in the formation of the meromycolic acid. The disruption of the interactions between Had complexes and SAM-dependent methyl transferases would explain the mycolic acid profile of 15- and 16-treated mycobacteria: less mycolates and accumulation of the unsaturated precursors. Why 15 and 16 are more active than TAC remains unknown, but they could be more stable and/or more efficient perturbagenic molecules than TAC. The ability of 15 to inhibit keto-mycolic acid biosynthesis could be explained by a greater capacity to disrupt specific interactions.
a modest, but reproducible, accumulation of unsaturated mycolic acids, presumably due to the inhibition of SAM-dependent methyltransferases involved in introducing cyclopropane rings on the meromycolates, as reported previously in other mycobacterial strains. While this effect was very limited following TAC LOUREIRIN-B treatment, accumulation of unsaturated keto-mycolic acid precursors was more pronounced after exposure to higher concentrations of 15 and 16, consistent with our previous observations using the SRI-224 analogue. One would expect that all mycolic acid subspecies would be inhibited concomitantly, leading to complete cessation in mycolic acid biosynthesis, as is seen with specific FAS-II inhibitors such as INH or ETH. FAS-II components are all essential and known to participate in the meromycolyl-ACP elongation steps, and it is during this process that the specific methyltransferases involved in the formation of the meromycolic acid are likely to operate. That keto-mycolic acids are refractory to TAC inhibition is somehow intriguing and argues against the HadABC being directly targeted by TAC. Supporting this view, both the recent study by Belardinelli and Morbidoni and the present study failed to identify mutations within the HadB subunit bearing the catalytic activity. Mutations only occurred in the HadA and HadC subunits, perhaps 4-(Benzyloxy)phenol stabilizing the complex and presenting the growing acyl-ACP substrate to HadB, thought to be the enzymatically active component of the complex. Elegant work demonstrated that the FAS-II system of M. tuberculosis is organized in specialized interconnected complexes composed of the condensing enzymes, dehydratase heterodimers and the methyltransferases. This led the authors to propose that because the interactions amongst these enzymes are crucial and their disruption detrimental for M. tuberculosis survival, the protein interactions could represent attractive drug targets. Whether this is the case for TAC remains to be established experimentally but seems highly conceivable. Our results also emphasize the possibility that EthA-activated TAC and related analogues are likely to inhibit mycolic acid biosynthesis by physically altering the interactions of HadAB and HadBC dehydratases with other FASII components, particularly those involved in the formation of the meromycolic acid. The disruption of the interactions between Had complexes and SAM-dependent methyl transferases would explain the mycolic acid profile of 15- and 16-treated mycobacteria: less mycolates and accumulation of the unsaturated precursors. Why 15 and 16 are more active than TAC remains unknown, but they could be more stable and/or more efficient perturbagenic molecules than TAC. The ability of 15 to inhibit keto-mycolic acid biosynthesis could be explained by a greater capacity to disrupt specific interactions.