The second protocol concerns the determination of accuracy for positioning ligands in proteins active sites. This protocol was used to compare the two docking programs, SOL and the standard AutoDock 3.05. The first protocol showed a good to excellent quality in the SOL program for the selection of active inhibitors for four different target-enzymes from a large set of active and inactive ligands. The accuracy of CT99021 ligand positioning in the active sites of enzymes was defined by the root mean square deviation between ligand docked poses and experimental ligand poses taken from the Protein Data Bank. The grid of potentials representing thrombin-ligand interactions was calculated separately using the SOL_GRID program, before the initiation of the docking procedure. Throughout the docking studies, all ligands were considered fully flexible �C i.e., all topologically available torsional degrees of freedom were unfrozen and allowed to VE-821 rotate freely, directed only by ligand internal energy preferences in the frame of MMFF94. Bond lengths and valence angles were frozen in the course of the docking procedure. A fast decrease of preformed thrombin activity rises is vital in acute situations. Thus, it is reasonable in such cases to intravenously administer direct thrombin inhibitors to block hypercoagulation as quickly as possible. Our aim was to 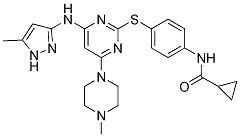 design new thrombin inhibitors for intravenous administration, whereby inhibitors can get directly to blood plasma where thrombin works. Thus, bioavailability was not an issue, and we were not restricted to ligands with low basicity in their P1 fragments. We have shown before that moderate plasma dilution in vitro with different artificial PSS produced hypercoagulation changes in the coagulation system. This fact suggests that plasma dilution, especially by crystalloid PSS, could also be a risk factor for the induction of thrombotic states during moderate hemodilution in vivo. The development of hypercoagulation has been shown to correlate with the infusion of large volumes of crystalloid solutions in patients. At present, the mechanism of this phenomenon is not clear; however, many investigators propose that during moderate hemodilution, the coagulation system is more sensitive to decreasing concentrations of coagulation inhibitors than to dilution of procoagulant factor precursors that are present in the blood in abundance. To prevent the development of hemodilutional hypercoagulation, we supplemented a crystalloid PSS with DTI. It was shown that the natural thrombin inhibitor antithrombin III could be used for this purpose. However, this inhibitor is isolated from human plasma and is thus very expensive and not completely safe with regard to the transmission of viral infections. Small molecule synthetic thrombin inhibitors are more suitable for this purpose. To be used in PSS, these inhibitors should be not only highly effective and safe, but also stable in aqueous solutions. The development of this kind of inhibitor was one of the objectives of our study. A majority of successful thrombin inhibitors have positively charged or neutral but easy polarizable P1 fragments. During thrombin-inhibitor complex formation, the P1 moiety of the inhibitor is located in the thrombin active site within a narrow cavity, exposing the carboxyl side chain of the Asp189 residue on its bottom. The severe spatial restrictions dictate the small size and hydrophobic nature of the P2 inhibitor position. In contrast, the restrictions on the P3 site are not as stringent because the corresponding binding site in the thrombin molecule is broad and exposed to the solvent.
design new thrombin inhibitors for intravenous administration, whereby inhibitors can get directly to blood plasma where thrombin works. Thus, bioavailability was not an issue, and we were not restricted to ligands with low basicity in their P1 fragments. We have shown before that moderate plasma dilution in vitro with different artificial PSS produced hypercoagulation changes in the coagulation system. This fact suggests that plasma dilution, especially by crystalloid PSS, could also be a risk factor for the induction of thrombotic states during moderate hemodilution in vivo. The development of hypercoagulation has been shown to correlate with the infusion of large volumes of crystalloid solutions in patients. At present, the mechanism of this phenomenon is not clear; however, many investigators propose that during moderate hemodilution, the coagulation system is more sensitive to decreasing concentrations of coagulation inhibitors than to dilution of procoagulant factor precursors that are present in the blood in abundance. To prevent the development of hemodilutional hypercoagulation, we supplemented a crystalloid PSS with DTI. It was shown that the natural thrombin inhibitor antithrombin III could be used for this purpose. However, this inhibitor is isolated from human plasma and is thus very expensive and not completely safe with regard to the transmission of viral infections. Small molecule synthetic thrombin inhibitors are more suitable for this purpose. To be used in PSS, these inhibitors should be not only highly effective and safe, but also stable in aqueous solutions. The development of this kind of inhibitor was one of the objectives of our study. A majority of successful thrombin inhibitors have positively charged or neutral but easy polarizable P1 fragments. During thrombin-inhibitor complex formation, the P1 moiety of the inhibitor is located in the thrombin active site within a narrow cavity, exposing the carboxyl side chain of the Asp189 residue on its bottom. The severe spatial restrictions dictate the small size and hydrophobic nature of the P2 inhibitor position. In contrast, the restrictions on the P3 site are not as stringent because the corresponding binding site in the thrombin molecule is broad and exposed to the solvent.