These findings are in agreement with those of that showed that NF-kB DNA binding activity did not change in response to heat stress in rats; yet, they are AbMole Folinic acid calcium salt pentahydrate somewhat surprising since NF-kB has been implicated as the main TF that regulates cytokine mRNA accumulation during inflammation. Moreover, both TNF-a liver mRNA accumulation and plasma concentrations were not affected by the heat insult in contrast to other studies that reported elevated concentrations of this cytokine after HS. Although these discrepancies between studies may be partly explained by species differences in HS responses. We hypothesize that activation of NF-kB and increased concentration of TNF-a depend on the duration and severity of the heat insult. An in silico experiment with a more severe heat insult illustrates that our model supports this hypothesis with a molecular mechanism capable of explaining the different behaviors, encompassing the apparently contradictory results from the literature. AP-1 is revealed here as the main transcription factor activated in the liver during HS recovery and it appears to be responsible for the high expression of many pro-inflammatory cytokines in spite of the low activation of NF-kB. The fact that JUN and FOS liver mRNA accumulation is lower in TNFR KO mice at baseline indicates that the differences in TNFR KO responses are not only due to differences in heating and cooling but a direct effect of the lack of TNF signaling. It is intriguing to speculate that lower JUN and FOS mRNA accumulation may be a direct consequence of the absence of a TNF autocrine cascade effect on AP-1 activation, which has been shown to be triggered in hepatocytes in response to TNF. IL1b, IL-1RI, IL-6, and IL-6R also presented lower mRNA accumulation in normothermic conditions for TNFR KO, which can be explained by the lower activation of AP-1. However, we reject the hypothesis that the changes in liver gene expression in our model were only related to the TNF autocrine cascade since we failed to detect changes in liver TNF mRNA accumulation in WT mice at any time point of HS recovery. Rather, our data suggest that delayed liver gene expression was due to a combination of factors that include: 1) lower initial expression; 2) indirect effect of other genes that activate liver AP-1 that also showed a delayed response ; 3) lower core temperature of TNFR KO mice during heat exposure. Unfortunately, it was not possible to normalize the heating responses between genotypes since the absence of TNF signaling induced a downward shift in the temperature set point, thus causing the KO mice to regulate Tc and metabolic rate differentially from WT mice. AP-1 may deactivate the heat shock response during stress recovery by hyperphosphorylating HSF-1 to inhibit its function ; hence, robust activation of AP-1 could lead to considerably decreased HSP70 gene expression in response to HS. Since 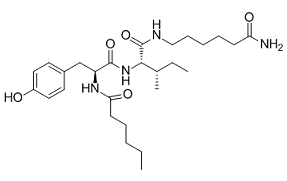 HSP70 is concomitantly inhibiting the activation of NF-kB, HSP70 downregulation could exacerbate the situation leading to a lethal concentration of proinflammatory NFkB dependent genes, such as TNF-a. Therefore, AP-1 and its related cofactor genes are revealed here as promising targets for future therapeutic intervention to AbMole 4-(Benzyloxy)phenol accelerate HS recovery. In contrast, HSPs exert an anti-inflammatory effect by inhibiting the translocation of NF-kB to the nucleus; therefore, it is expected that drug-induced increases in HSP70 gene expression prior to or following HS collapse may attenuate the heat-induced SIRS in the liver and perhaps other organ systems.
HSP70 is concomitantly inhibiting the activation of NF-kB, HSP70 downregulation could exacerbate the situation leading to a lethal concentration of proinflammatory NFkB dependent genes, such as TNF-a. Therefore, AP-1 and its related cofactor genes are revealed here as promising targets for future therapeutic intervention to AbMole 4-(Benzyloxy)phenol accelerate HS recovery. In contrast, HSPs exert an anti-inflammatory effect by inhibiting the translocation of NF-kB to the nucleus; therefore, it is expected that drug-induced increases in HSP70 gene expression prior to or following HS collapse may attenuate the heat-induced SIRS in the liver and perhaps other organ systems.