A specialized cellular substructure has been identified in which such decisions may be made, but on what basis is still unclear. One can envisage Ginsenoside-F4 termination of the exchange reaction based on generation of a true DM-mediated “compact” conformation. The answer may lie in elucidating the molecular details of the resolution of the tetramolecular complex. The “compare-exchange” mechanism proposed here for DM mechanism might also be important for antigen processing via the MHC class I pathway. MHC class I processing involves proteins structurally related to MHCII, and both classes of MHC molecules undergo peptide-dependent conformational change. Furthermore, the majority of MHC class I alleles requires the intervention of the tapasin-ERp57 heterodimer to optimize their peptide cargo. The “compare-exchange” mechanism may provide additional insights into the biology of MHC antigen processing, and may also be generally applicable to other biological systems in which protein receptors must bind diverse yet structurally related ligands. An increasing number of reports have highlighted the function of the primary cilium in the control of several physiological processes. The PC is a hair-like cellular extension found at the surface of most vertebrate cells. This sophisticated microtubulebased organelle has been shown to sense multiple mechanical and chemical stimuli from the environment and to elicit specific cellular responses, which play crucial roles in embryonic development and homeostatic processes in adulthood. The PC has also recently been implicated in the regulation of cell cycle progression and, as a consequence, a lack of PC was associated with increased proliferation. PC formation takes place in quiescent or differentiated cells. PCs are assembled from the mother centriole of the unique centrosome present in these cells, which therefore corresponds to the basal body of PC. which forms the skeleton of this “antenna” like extension of the plasma membrane. Whereas the basal body shares many properties with classical centrosomes, made of two Catharanthine sulfate centrioles and of a pericentriolar matrix, the axoneme represents a unique domain, characterized by the exclusion  of many proteins and the enrichment of specific soluble, cytoplasmic, as well as membrane-associated components.
of many proteins and the enrichment of specific soluble, cytoplasmic, as well as membrane-associated components.
Monthly Archives: June 2019
As evidenced by the ability of the mitochondrial poison 3-nitropropionic acid to mimic
Apparent relationship between aggregate 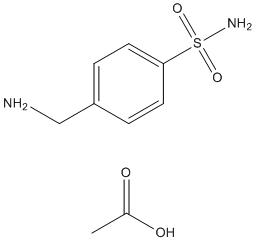 size and “toxic surface area” deserves further investigation, especially with regard to intracellular amyloidogenic proteins in compound aggregated states such as inclusion bodies and aggresomes. Alternatively, we cannot exclude the possibility that scFv-6E may Mechlorethamine hydrochloride stabilize a recently described oligomeric species having fibrillar features, although the existence of such oligomers has yet to be correlated with cytopathology. On this subject, it is noteworthy that the fibrillogenesis of ataxin-3 is initiated by a monomeric nucleus and grows by monomer addition rather than through oligomers in solution, and a similar mechanism may hold true for mutant httex1 as well. Paradoxically, viable cells containing intracellular aggregates significantly outnumbered those that died with aggregates in our cytotoxicity experiments, demonstrating that aggregates per se are not instantly toxic to ST14A striatal-derived cells. This observation is consistent with previous reports demonstrating a penchant for inclusion bodies to be protective, a fact which has called into question how visible protein Labetalol hydrochloride aggregation can be associated with neuropathological abnormalities even though aggregates themselves are not universally predictive of neurodegeneration. Using intrabodies to alter the kinetics of amyloidogenesis, our data illustrates that the overall speed of aggregate formation bears more relevance to the severity of intracellular dysfunction than whether or not inclusions are present at any given time point. Indeed, time-lapse microscopy in a PC12 cell model of HD has revealed that aggregation tends to occur rapidly in cells that die with inclusions and much slower in cells that survive with inclusions. We conclude that the rate of aggregation likely influences the magnitude of cellular dysfunction elicited by aggregation, such as oxidative stress in the case of mutant httex1. Hence, escalating the formation of httex1 aggregates with a fibrilspecific intrabody heightens oxidative stress and results in greater toxicity in ST14A striatal cells. In contrast, suppressing aggregation with an intrabody that shields the amyloidogenic N-terminus of mutant huntingtin in fact reduces oxidative stress and toxicity in these cells. Importantly, oxidative stress complements several prevailing models of HD pathogenesis, including excitoxicity, mitochondrial dysfunction, and inflammation.
size and “toxic surface area” deserves further investigation, especially with regard to intracellular amyloidogenic proteins in compound aggregated states such as inclusion bodies and aggresomes. Alternatively, we cannot exclude the possibility that scFv-6E may Mechlorethamine hydrochloride stabilize a recently described oligomeric species having fibrillar features, although the existence of such oligomers has yet to be correlated with cytopathology. On this subject, it is noteworthy that the fibrillogenesis of ataxin-3 is initiated by a monomeric nucleus and grows by monomer addition rather than through oligomers in solution, and a similar mechanism may hold true for mutant httex1 as well. Paradoxically, viable cells containing intracellular aggregates significantly outnumbered those that died with aggregates in our cytotoxicity experiments, demonstrating that aggregates per se are not instantly toxic to ST14A striatal-derived cells. This observation is consistent with previous reports demonstrating a penchant for inclusion bodies to be protective, a fact which has called into question how visible protein Labetalol hydrochloride aggregation can be associated with neuropathological abnormalities even though aggregates themselves are not universally predictive of neurodegeneration. Using intrabodies to alter the kinetics of amyloidogenesis, our data illustrates that the overall speed of aggregate formation bears more relevance to the severity of intracellular dysfunction than whether or not inclusions are present at any given time point. Indeed, time-lapse microscopy in a PC12 cell model of HD has revealed that aggregation tends to occur rapidly in cells that die with inclusions and much slower in cells that survive with inclusions. We conclude that the rate of aggregation likely influences the magnitude of cellular dysfunction elicited by aggregation, such as oxidative stress in the case of mutant httex1. Hence, escalating the formation of httex1 aggregates with a fibrilspecific intrabody heightens oxidative stress and results in greater toxicity in ST14A striatal cells. In contrast, suppressing aggregation with an intrabody that shields the amyloidogenic N-terminus of mutant huntingtin in fact reduces oxidative stress and toxicity in these cells. Importantly, oxidative stress complements several prevailing models of HD pathogenesis, including excitoxicity, mitochondrial dysfunction, and inflammation.
Likewise neuronal protein inclusions are clinically binds Tat-SF1
Indeed, another transcription-splicing coupling factor, TCERG1 directly through multiple interactions with FF domains in the former. Insight into the role of Diperodon Tat-SF1 in the HIV-1 lifecycle has previously been limited to immunodepletions and in vitro analyses or transient overexpression experiments. In this manuscript, we present studies that Orbifloxacin utilize RNA interference to reevaluate Tat-SF1��s role in Tat transactivation and HIV-1 replication in vivo. We found that Tat-SF1 depletion did not affect transcription from the HIV-1 LTR and did not alter the overall level of viral transcripts; however, Tat-SF1 depletion resulted in a significant decrease in viral replication. This study demonstrates that the major effect upon knockdown of Tat-SF1 was a change in the ratio of unspliced to fully spliced HIV-1 RNAs. Based on our data, we propose a novel activity for Tat-SF1 as a post-transcriptional regulator of viral pre-mRNAs. Abnormal aggregation of polypeptides into amyloid-like fibrils is associated with more than 20 known human disorders collectively referred to as protein misfolding or conformational diseases. Despite little shared sequence homology, amyloid-forming polypeptides show a common propensity to misfold into highlyordered polymers that are rich in fibrillar b-sheet structure. Distinct amyloidogenic polypeptides are genetically implicated in the progression of human neurodegenerative disorders, including Alzheimer��s, Parkinson��s, polyglutamine, and prion diseases. Human polyglutamine disorders such as Machado-Joseph disease and Huntington��s disease are caused by aberrant codon expansion of CAG trinucleotide tracts within unrelated genes encoding polyglutamine-domain proteins. In HD, expansions beyond 37 consecutive glutamines within the huntingtin protein confer a toxic gain-of-function phenotype related to its intracellular aggregation in neurons. Proteinaceous huntingtin aggregates are diagnostic hallmarks of HD neuropathology and coincide with neurological symptoms in humans as well as in transgenic models of the disease. These intracellular aggregates are composed 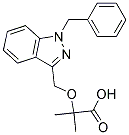 chiefly of polyglutamine-containing amino-terminal fragments of huntingtin that arise by proteolysis. Consequently, the first exon of the human Huntingtin gene containing an expanded CAG repeat is sufficient to induce HDlike pathology, including intracellular aggregates and neurodegeneration, in transgenic mice models.
chiefly of polyglutamine-containing amino-terminal fragments of huntingtin that arise by proteolysis. Consequently, the first exon of the human Huntingtin gene containing an expanded CAG repeat is sufficient to induce HDlike pathology, including intracellular aggregates and neurodegeneration, in transgenic mice models.