This irregular TCF/LEF activation is independent of Wnt receptor activation; however, changes in the homeostasis of cell lines bearing an APC mutation as a result of the effect of different Wnt inhibitors have been described. Based on the methylation analysis of macrodissected samples, it has been described that in colorectal carcinogenesis SFRP1 promoter is epigenetically silenced. In this study, we aim to examine the protein expression and methylation patterns of myofibroblast-derived SFRP1 in NAT and CRC tissues, and to demonstrate the effect of SFRP1 protein on HCT116 CRC cell line as a potential model of paracrine inhibition of the Wnt pathway in colorectal carcinoma. Wnt signaling is a major regulator of 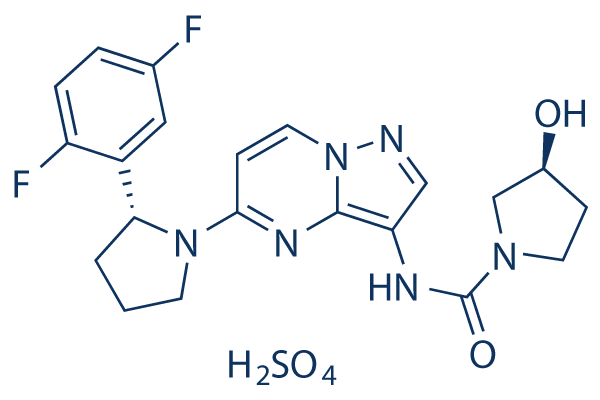 a variety of cellular processes during Masitinib embryonic development and promotes tissue homeostasis in the adult. Wnts are secreted lipid-modified glycoproteins regulating a wide range of cellular behavior including differentiation, proliferation, migration, survival, Vorinostat polarity and stem cell self-renewal. Altered Wnt signaling may contribute to the development of several disorders including cancer. The canonical/b-catenin pathway is the most extensively studied Wnt signaling mechanism, which is triggered by Wnt binding to a member of the Frizzled receptor family and co-receptors. This results in the recruitment of Dishevelled to Frizzled and Axin to phosphorylated LRP5/6, leading to the dissociation of a b-catenin degradation complex. In the absence of Wnt this complex mediates the sequential phosphorylation of b-catenin, causing its ubiquitination and proteasomal degradation. Wnt stimulation allows the accumulation of hypophosphorylated b-catenin in the cytosol and its translocation into the nucleus, where it binds to TCF/LEF and promotes the expression of Wnt/b-catenin target genes. Constitutive activation of this pathway is commonly present in many types of cancer. Non-canonical Wnt-signaling pathways are transduced by Frizzleds and/or other Wnt receptors or co-receptors. Several non-canonical Wnt signaling mechanisms have been reported to inhibit the b-catenin pathway by decreasing b-catenin/TCF association with DNA or increasing b-catenin turnover. SFRPs comprise a family of five proteins in mammals that were first identified as antagonists of the Wnt/b-catenin pathway during embryonic development. SFRPs possess a remarkable range of biological activities, including tumor suppression. This is also strengthened by epigenetic silencing of SFRP gene expression in a wide variety of cancers, and supported by the observation that restoration of expression is suppressive of the tumor phenotype. By contrast, SFRP overexpression has been observed in some of the same malignancies. Consistent with this duality, SFRP1 showed a biphasic effect on b-catenin stabilization elicited by Wingless, increasing b-catenin protein levels at low SFRP1 concentrations, but inhibiting it at high concentrations. In different cellular contexts, SFRP1 has been shown either to increase or decrease b-catenin stabilization. Furthermore, another study suggested that SFRP1 could stimulate the Wnt/calcium pathway via Frizzled-2 independently of endogenous Wnts. Regarding field cancerization, methylation of hMLH1, CDKN2A/P16 and SFRP1 has been recently indicated to be associated with malignant transition in endometrial cancer. In our study, the apoptotic effect of SFRP1 protein was demonstrated by administering low doses of rhSFRP1 on HCT116 CRC cell line. In HCT116 cells rhSFRP1 protein caused a measurable increase in apoptosis. We investigated the role of SFRP1 regarding the stromaepithelium interaction in CRC and NAT areas. SFRP1 protein is a well-known intercellular inhibitor of Wnt pathway which regulates the epithelial proliferation as autocrine and/or paracrine signal.
a variety of cellular processes during Masitinib embryonic development and promotes tissue homeostasis in the adult. Wnts are secreted lipid-modified glycoproteins regulating a wide range of cellular behavior including differentiation, proliferation, migration, survival, Vorinostat polarity and stem cell self-renewal. Altered Wnt signaling may contribute to the development of several disorders including cancer. The canonical/b-catenin pathway is the most extensively studied Wnt signaling mechanism, which is triggered by Wnt binding to a member of the Frizzled receptor family and co-receptors. This results in the recruitment of Dishevelled to Frizzled and Axin to phosphorylated LRP5/6, leading to the dissociation of a b-catenin degradation complex. In the absence of Wnt this complex mediates the sequential phosphorylation of b-catenin, causing its ubiquitination and proteasomal degradation. Wnt stimulation allows the accumulation of hypophosphorylated b-catenin in the cytosol and its translocation into the nucleus, where it binds to TCF/LEF and promotes the expression of Wnt/b-catenin target genes. Constitutive activation of this pathway is commonly present in many types of cancer. Non-canonical Wnt-signaling pathways are transduced by Frizzleds and/or other Wnt receptors or co-receptors. Several non-canonical Wnt signaling mechanisms have been reported to inhibit the b-catenin pathway by decreasing b-catenin/TCF association with DNA or increasing b-catenin turnover. SFRPs comprise a family of five proteins in mammals that were first identified as antagonists of the Wnt/b-catenin pathway during embryonic development. SFRPs possess a remarkable range of biological activities, including tumor suppression. This is also strengthened by epigenetic silencing of SFRP gene expression in a wide variety of cancers, and supported by the observation that restoration of expression is suppressive of the tumor phenotype. By contrast, SFRP overexpression has been observed in some of the same malignancies. Consistent with this duality, SFRP1 showed a biphasic effect on b-catenin stabilization elicited by Wingless, increasing b-catenin protein levels at low SFRP1 concentrations, but inhibiting it at high concentrations. In different cellular contexts, SFRP1 has been shown either to increase or decrease b-catenin stabilization. Furthermore, another study suggested that SFRP1 could stimulate the Wnt/calcium pathway via Frizzled-2 independently of endogenous Wnts. Regarding field cancerization, methylation of hMLH1, CDKN2A/P16 and SFRP1 has been recently indicated to be associated with malignant transition in endometrial cancer. In our study, the apoptotic effect of SFRP1 protein was demonstrated by administering low doses of rhSFRP1 on HCT116 CRC cell line. In HCT116 cells rhSFRP1 protein caused a measurable increase in apoptosis. We investigated the role of SFRP1 regarding the stromaepithelium interaction in CRC and NAT areas. SFRP1 protein is a well-known intercellular inhibitor of Wnt pathway which regulates the epithelial proliferation as autocrine and/or paracrine signal.
Monthly Archives: July 2019
These effects are the result of AP expression as an inhibitor for multiple M10 proteases under physiological conditions
These results suggest that serralysin-mediated ENaC activation requires active protease and that modulation of these protease activities could potentially be leveraged to effectively reduce the virulence associated with bacterial metalloprotease production and secretion. Proteolytic activation of ENaC has been postulated to play a key role in both normal and disease physiologies in the airway. As such, it is possible that both endogenous and exogenous proteases may play a role in establishing and remodeling the airway environment. Here we demonstrate that multiple members of the serralysin metalloprotease family are capable of activating ENaC. These data suggest that ENaC may serve as a target for the serralysin virulence factors from multiple human pathogens. Further, the Pseudomonas aeruginosa AprI, alkaline protease inhibitor can be effectively used to block the in vitro activities of purified serralysin proteases and reverse their effects in physiological experiments on cultured and primary epithelial cells. Our previous studies showed that ENaC can be activated by the addition of AP at the apical surface of cultured and primary epithelial cells. This activation may contribute to the virulence of Pseudomonas by remodeling the local airway environment to be more Vismodegib favorable for bacterial adhesion and subsequent colonization. The current study demonstrates that this activation is more general to this class of bacterial exoproteases, as serralysin from Serratia marcescens is similarly capable of activating ENaC. This activation is slow when compared to trypsin under maximal stimulating conditions. The slow activation of ENaC 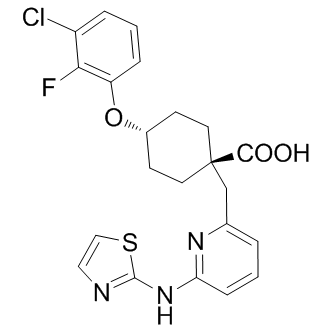 by both AP and SmP suggest that the physical basis of activation may also be similar for both proteases. However the kinetics of ENaC activation were slightly accelerated in SmP treated epithelia compared to AP, in line with the biophysical characterization of the protease activities. Binding of the inhibitor to AP and SmP is tight, as measured in vitro using purified proteins, and completely abolishes protease activity, consistent with prior reports of binding between the protease and inhibitor. This tight in vitro binding is observed as a complete loss of protease-induced ENaC current in two different model epithelia. This inhibition provides evidence that the activation of ENaC is mediated through cleavage of a host protein by the bacterial protease. The coincident inhibition of protease activity and loss of ENaC activation suggests that the observed activation is occurring through one or more cleavage events and is not mediated by other non-catalytic binding or protein-protein interactions. The AP and SmP mediated activation is slow when compared to that elicited by trypsin. The kinetics of ENaC activation by AP and SmP are slowed by,3.5 to 20 fold when compared to trypsin in the two cell lines. Though previous studies have demonstrated that cleavage of the c-subunit is required for AP induced ENaC activation, it is not immediately clear why the activation kinetics vary between the trypsin and the bacterial proteases. The relatively slow and submaximal activation may arise from conformational constraints limiting GDC-0879 purchase access to one or more cleavage sites in the ENaC ectodomain. This would be consistent with a model wherein the mechanisms of ENaC cleavage and activation did not co-evolve with the bacterial proteases. Alternatively, this slow activation may be the result of indirect activation via an additional protease-sensitive pathway. Further work to evaluate these differences in activation kinetics is ongoing. Both the AP and SmP proteases have been implicated in bacterial virulence. Previous studies have suggested that AP is associated with exacerbations in CF and complications in treating Pseudomonas.
by both AP and SmP suggest that the physical basis of activation may also be similar for both proteases. However the kinetics of ENaC activation were slightly accelerated in SmP treated epithelia compared to AP, in line with the biophysical characterization of the protease activities. Binding of the inhibitor to AP and SmP is tight, as measured in vitro using purified proteins, and completely abolishes protease activity, consistent with prior reports of binding between the protease and inhibitor. This tight in vitro binding is observed as a complete loss of protease-induced ENaC current in two different model epithelia. This inhibition provides evidence that the activation of ENaC is mediated through cleavage of a host protein by the bacterial protease. The coincident inhibition of protease activity and loss of ENaC activation suggests that the observed activation is occurring through one or more cleavage events and is not mediated by other non-catalytic binding or protein-protein interactions. The AP and SmP mediated activation is slow when compared to that elicited by trypsin. The kinetics of ENaC activation by AP and SmP are slowed by,3.5 to 20 fold when compared to trypsin in the two cell lines. Though previous studies have demonstrated that cleavage of the c-subunit is required for AP induced ENaC activation, it is not immediately clear why the activation kinetics vary between the trypsin and the bacterial proteases. The relatively slow and submaximal activation may arise from conformational constraints limiting GDC-0879 purchase access to one or more cleavage sites in the ENaC ectodomain. This would be consistent with a model wherein the mechanisms of ENaC cleavage and activation did not co-evolve with the bacterial proteases. Alternatively, this slow activation may be the result of indirect activation via an additional protease-sensitive pathway. Further work to evaluate these differences in activation kinetics is ongoing. Both the AP and SmP proteases have been implicated in bacterial virulence. Previous studies have suggested that AP is associated with exacerbations in CF and complications in treating Pseudomonas.
Interspecies differences in terms of immune response or ENS phenotype can therefore limit the extrapolation
Using an ex vivo human organ culture model, we showed that the invasive but not the non invasive strain of S. flexneri induced significant desquamation of the IEB which was significantly reduced following infection with SepA deficient S. flexneri strains. In addition, S. flexneri also induced rapid neuronal morphological alterations suggestive of cell death. These alterations were associated with a significant increase in the proportion of VIP-IR neurons. Morphological changes induced by S. flexneri but not the increase in VIP-IR were blocked by the NMDA receptor antagonist MK-801. In addition, the absence of data describing early effects of shigellosis in humans is mainly due to the lack or scarse material from patients infected with S. flexneri prior to the development of their disease and symptoms. In this context, our model has the advantage to allow the characterization of early events following S. flexneri infection in the human colon upon both the IEB and the ENS. It also presents the advantage over reductionist cellular models to integrate various components of the mucosa such as IEC, submucosal neurons and resident inflammatory cells. One major finding of the study was the presence of a massive desquamation of surface epithelium induced by S. flexneri, as early as three hours post-infection. These results are reminiscent of observations obtained in vivo, both in animal models and in patients with shigellosis. In particular, human intestinal segments grafted into SCID mice infected with S. flexneri showed focal desquamation of the epithelial lining without ulcerations, similar to our C188-9 observations. Interestingly, the lesions of the mucosa were not reduced in polynuclear deficient mice. This is consistent with our observations since no recruitment of neutrophils can occur in the colonic explants used in our experiments. In addition, damages of the mucosa occurred in area devoid of M cells suggesting that, in our model, S. flexneri does not require entry through Peyer’s patches. A striking finding was the rapidity of the lesions induced by S. flexneri which occurred within 3 h post-infection. This could be due to the high inoculum of bacteria used and the absence of peristaltism and secretory response in our ex vivo model. In addition, a basal inflammation in the explant, probably due to hypoxic conditions generated by the culture and absence of vascularisation, might also have potentiated the mucosal alterations induced by S. flexneri. Interestingly, no bacteria was identified within intestinal epithelial cells, although they were present in some immune cells, but mainly directly beneath the shedding epithelial layer. This observation is also consistent with ND-630 in vivo animal studies showing few bacteria in the epithelium but localized mainly in the lamina propria and submucosa. Our study shows that growth-induced solid stress can affect cell phenotype, and suggests that there may be a ‘‘dynamic equilibrium’’ of proliferation and apoptosis that maintains tumor size in the plateau phase, as proposed by Holmgren et al. If only a small amount of IL-4 or 13 cross the placenta, then the level phosphorylation may be very low, and not detectable by Western Blot.
Galloyl catechins modify the staphylococcal phenotype through direct interactions with cellular proteins
It is surprising, therefore, that both microarray and qRT-PCR data showed that the capacity of the EC-ECg combination to modulate EMRSA-16 gene expression was reduced compared to ECg alone; this is likely to indicate that the position adopted within the bilayer by ECg, in the absence of EC, maximizes the strength of the signals governing reconfiguration of cytoplasmic membrane architecture. Thus, although vraX was strongly up-regulated by the catechin combination as judged by qRT-PCR, it was significantly less than that found with ECg alone. Similarly, transcription of dltA, mecA, fmtA and isaA were significantly upregulated by ECg but modulation of these genes failed to reach levels of statistical significance when cells were exposed simultaneously to ECg and EC. Differences in the capacity of ECg and EGCg to interact with lipid bilayers suggested that changes to the B-ring hydroxylation pattern could influence the bioactivity of galloyl catechins. It was therefore surprising that 2 failed to induce ‘‘over-compensation’’ of membrane fluidity after 4 h incubation in a similar way to ECg and compounds 1 and 3; there appears to be no obvious reason for this highly reproducible finding as in other respects ECg, 1, 2 and 3 represent a series of compounds with progressively enhanced membrane-perturbing properties. Although we have previously found little or no evidence, it remains a possibility that the profound effect of 2 on virulence gene expression and relatively low impact on membrane fluidity is due to interactions with elements of the stress regulon or other two-component systems; this will be investigated in future studies. Compound 3, carrying no hydroxyls on the Bring, was associated with an increased oxacillin susceptibilitymodifying capacity, and induced both a highly fluid staphylococcal cytoplasmic membrane comparable to the ECg/EC combination and a level of gene modulation higher than other molecules in this series,JNJ-42153605 supporting the contention that progressive removal of –OH groups from the B-ring increased membrane interactions and bioactivity. In this context, 3 had a greater impact on the biophysical properties of LPG:PG:CL vesicles than ECg, strongly increasing the lipid order of fluid bilayers from a location close to the lipid/water interface. Compounds 4 and 5, in which all hydroxyl groups had been excluded from the A- and B-rings, possessed clear antibacterial activity and were poor resistance modifiers compared to ECg and analogs 1–3. Compound 5 impacted primarily on EMRSA-16 by disrupting the integrity of the cytoplasmic membrane, reflected in the induction of a different transcriptomic profile compared to molecules 1–3. The large majority of the genes selected for qRTPCR analysis LJI308 were significantly down-regulated at concentrations below the MIC, reflecting the overt antibacterial properties of this analog. Compound 5 dramatically altered the thermal characteristics of MLVs modelled on the S. aureus cytoplasmic membrane, shifting Tm to a lower temperature and creating a second peak of transition; 5 also appeared to render cells non-viable from a more superficial location in the membrane compared to ECg.This study has shown that progressive removal of hydroxyl groups from ECg effects a transition from molecules eliciting an extremely weak antibacterial effect but characterized by a capacity to induce a b-lactam-susceptible phenotype to overtly antibacterial compounds with limited b-lactam-resistance modifying properties.
There may be insufficient GSH for S-glutathionylation to produce normal regulation in such experiments
The balance between the formation of mixed protein-glutathione disulfides verses protein-protein disulfides depends on two factors: the relative redox potentials between cysteine thiols and GSH and the relative concentrations of reactant and product species. Previous findings have suggested that Kv4 channels, unlike Kv1.4 channels, do not produce redox-sensitive A-type K + currents. The A-type currents generated in oocytes by heterologous expression of Kv4 mRNA alone or poly-A mRNA from rat thalamus are insensitive to H2O2. Moreover, in hippocampal pyramidal neurons, the somatodendritic subthreshold A-type current mediated by Kv4 channels is also reportedly insensitive to oxidants. However, recent progress in our molecular understanding of the ISA channel complex challenges this overly simplistic conclusion. In addition to Kv4 pore-forming subunits, ISA channels contain notably two types of auxiliary subunits, the Kv channel-interacting proteins and dipeptidyl peptidase-like proteins. KChIP binding sequesters the Kv4 N-termini and effectively removes Ntype inactivation mediated by Kv4 subunits, meaning that in many neurons, ISA does not utilize N-type mechanisms. However, specific neuronal populations express two DPLP Nterminal variants that can independently confer N-type inactivation on the Kv4-KChIP-DPLP channel complex. The published literature has established that, unlike Kv1.4, the endogenous N-terminus of Kv4.2 produces fast inactivation that is insensitive to redox. Consequently, redox-sensitive A-type currents in neurons is thought to be likely expressed by Kv1.4 rather than Kv4 channels. Our heterologous expression studies strongly suggest that such interpretation should be reconsidered, since the Kv4-based subthreshold A-type current in neurons expressing DPP6a or DPP10a is likely also redox sensitive. Moreover, with a mixture of DPLPs being expressed in different neurons with different redox sensitivities, our results point to the importance of knowing the precise subunit SKF-86002 composition in order to predict the functional and regulatory properties of the native current. Furthermore, since DPP6a-conferred N-type inactivation of Kv4 channels is regulated via S-glutathionylation, follow-up studies to examine redox regulation of ISA in neurons or Kv4.2+KChIP3a+DPP6a currents in cultured mammalian cells must maintain normal intracellular GSH concentration to permit S-glutathionylation. This is an important consideration since, in the whole-cell patch configuration, the intracellular solutions dialyses out the intracellular milieu, and without GSH supplementation. Finally, our results emphasize the potential link between ISA, ROS, and oxidative stress-related disorders and the need for further studies. In neurons, ROS is robustly produced as a byproduct of normal aerobic metabolism and carefully kept in check by antioxidants such as GSH. At physiological concentration, ROS contributes to the induction and long-term potentiation,SMER 28 synaptic plasticity, learning and memory, and normal cognitive functions. Because ISA is also a major contributor to many of the same neurological phenomena modulated by ROS, our results would suggest that ROS may perform its function through reversible oxidation-reduction of DPP6a and DPP10a ISA auxiliary subunits. Under pathological conditions, an excessive accumulation of ROS results in neuronal oxidative stress, leading to apoptosis and reduced cognitive functions. An intriguing association between ROS and ISA has been reported in cerebellar granule cells where DPP6a is highly expressed. Exposure of CG cells to low external potassium concentrations leads to increased ROS levels, decreased cell viability, increased ISA amplitude, and slowing of inactivation kinetics. High-mobility group proteins are small DNA-binding proteins that serve an important role in transcriptional regulation.