To degrade LDs that are enriched with CE, however, lysosomal acid lipase, which has CE hydrolytic activity, may be involved, as it is for degradation of cholesterolloaded macrophages. For LDs containing CE and TG in comparable amounts, CE hydrolysis may be a prerequisite for effective TG degradation because CE may surround the TG core, forming concentric Perifosine layers on the surface. In this context, it is notable that the deficiency of lysosomal acid lipase that characterizes Wolman disease manifests as an accumulation of CE as well as TG. It was surprising that, upon treatment with translation inhibitors, TIP47 was recruited to the CE-rich LDs even though the total amount of TIP47 decreased drastically. TIP47 was previously shown to be recruited to TG-rich LDs induced by unsaturated fatty acids, but in such cases the overall expression of TIP47 also increased. The present result Carfilzomib indicates that TIP47 recruitment to LDs does not depend on the increased expression of TIP47 or on the composition of the lipid esters in LDs; rather, it is directly related to the increment of lipid esters. On the other hand, the increased recruitment of TIP47 to LDs should reduce TIP47 in the soluble cytoplasmic fraction, especially when the total amount is downregulated. Although the non-LD function of TIP47 remains controversial, it must be determined whether any result seen in the presence of translation inhibitors can be explained by a decrease in TIP47 in the cytoplasm. The phenomena observed in the present study need to be taken into account in  interpreting experimental results obtained using translation inhibitors. Yet the implications of this study are not limited to such artificial conditions, given that, in cells exposed to various stresses, protein synthesis is suppressed and LDs increase. LDs that increase in cultured cells under ER stress are enriched with CE. The detailed mechanism underlying CE-rich LD formation as well as the impact of this process are worthy of further studies in this context. Cyclin dependent kinases are a group of protein kinases which regulate different stages of the eukaryotic cell cycle. CDKs are also involved in the control of gene transcription, the processes that integrate extracellular and intracellular signals for the coordination of the cell cycle in response to environmental change, and apoptosis. Activation of CDKs usually occurs via phosphorylation of specific threonine residues by the CDKactivating kinase and binding to a cyclin protein. CDK4 plays a central role in the regulation of the G0-G1 phase of the cell and is required for the G1/S phase transition. CDK4 inactivates the retinoblastoma protein by phosphorylation. pRb is a negative regulator of the E2F family of transcription factors, hence phosphorylation of pRb results in the release of transcription factors which activate the expression of the S-phase genes. This process enables the cell to pass through the restriction point and results in the onset of the S-phase. Cell cycle regulators are frequently mutated in human cancers and due to their central role in G1 regulation CDKs offer attractive targets for therapeutic inhibition. The work of Yu et al. and Landis et al. suggests that inhibition of CDK4 might benefit patients with ErbB-2 initiated breast cancers. The CDK4/CyclinD1 complex as an anti-cancer drug target has been further validated in MCF-7 breast cancer cells. More than 20 small molecule inhibitors for CDKs are in clinical trials. For example, Flavopiridol is in clinical development for the treatment of different metastatic cancers. R-Roscovitine inhibits CDK2, CDK7 and CDK9 and is also in clinical trials.
interpreting experimental results obtained using translation inhibitors. Yet the implications of this study are not limited to such artificial conditions, given that, in cells exposed to various stresses, protein synthesis is suppressed and LDs increase. LDs that increase in cultured cells under ER stress are enriched with CE. The detailed mechanism underlying CE-rich LD formation as well as the impact of this process are worthy of further studies in this context. Cyclin dependent kinases are a group of protein kinases which regulate different stages of the eukaryotic cell cycle. CDKs are also involved in the control of gene transcription, the processes that integrate extracellular and intracellular signals for the coordination of the cell cycle in response to environmental change, and apoptosis. Activation of CDKs usually occurs via phosphorylation of specific threonine residues by the CDKactivating kinase and binding to a cyclin protein. CDK4 plays a central role in the regulation of the G0-G1 phase of the cell and is required for the G1/S phase transition. CDK4 inactivates the retinoblastoma protein by phosphorylation. pRb is a negative regulator of the E2F family of transcription factors, hence phosphorylation of pRb results in the release of transcription factors which activate the expression of the S-phase genes. This process enables the cell to pass through the restriction point and results in the onset of the S-phase. Cell cycle regulators are frequently mutated in human cancers and due to their central role in G1 regulation CDKs offer attractive targets for therapeutic inhibition. The work of Yu et al. and Landis et al. suggests that inhibition of CDK4 might benefit patients with ErbB-2 initiated breast cancers. The CDK4/CyclinD1 complex as an anti-cancer drug target has been further validated in MCF-7 breast cancer cells. More than 20 small molecule inhibitors for CDKs are in clinical trials. For example, Flavopiridol is in clinical development for the treatment of different metastatic cancers. R-Roscovitine inhibits CDK2, CDK7 and CDK9 and is also in clinical trials.
Monthly Archives: August 2019
Optimal peptide substrates stretch of emphasising the importance of exposed charged residues
Since this domain spans 84 aa, and in view of the difficulty of synthesizing large peptides, we decided to synthesize a 73 aa peptide spanning aa 25-97, which represents the sequence least similar to the non-interacting AnxA1. In conclusion, the present report demonstrates an extrahepatic physiological function of AnxA2 in regulating PCSK9’s ability to enhance the degradation of the LDLR, especially in adrenals and the digestive organs. Whether AnxA2 is implicated in the fine regulation of PCSK9 function during embryonic development or in some situations requiring high levels of cholesterol, such as in regenerating tissues, will need further studies. The ability of an AnxA2 peptide to inhibit PCSK9 function may be a prelude to the synthesis  of novel small molecule inhibitors of PCSK9. The IKK family of kinases consists of four family members, the canonical IKKa and IKKb, as well as two noncanonical family members, IKKe and TBK1. Together, this family of kinases regulates a myriad of critical cellular processes including inflammation, survival, proliferation, senescence, and autophagy. Consistent with these numerous functions, aberrant IKK signaling can result in susceptibility to diseases such as inflammatory disorders and cancer. The canonical IKK complex, which consists of IKKa, IKKb, and a regulatory subunit, NEMO, is a point of convergence for a variety of stimuli. Upon activation, the canonical IKKs, primarily IKKb, phosphorylate IkBa, the inhibitor of NF-kB, which promotes the ubiquitination and degradation of IkBa. The transcription factor NF-kB is then freed to accumulate in the FTY720 nucleus and U0126 109511-58-2 activate the transcription of a number of target genes involved in inflammatory and stress responses. In contrast to the canonical IKKs, IKKe and TBK1 are activated by a smaller subset of inflammatory stimuli, and are especially critical for antiviral responses. These kinases phosphorylate and activate the transcription factors IRF3, IRF7, and STAT1, promoting a Type 1 interferon response. These kinases also activate NF-kB, but the mechanism by which this occurs in unclear since they do not phosphorylate both of the serines on IkBa which are required for IkBa degradation. IKKe and TBK1 can also promote oncogenesis. For example, IKKe is overexpressed in some breast and ovarian cancers, and TBK1 was recently shown to be important for Ras-induced cell transformation. In spite of the important role these kinases play in both inflammatory and oncogenic signaling, few inhibitors have been identified. BX-795, a small molecule inhibitor of 3phosphoinositide-dependent protein kinase 1, inhibits both IKKe and TBK1 at low nanomolar concentrations in vitro. However, BX795 lacks selectivity as 16 out of 76 tested kinases were inhibited by BX-795 in the nM range. It was also recently shown that a series of azabenzimidazole derivatives inhibits these kinases in the low nM range, but 6 of 79 kinases tested using one of these compounds were inhibited in a range within 10-fold of TBK. These results suggest that IKKe and TBK1 are suitable targets for small molecule inhibitor development, but the need for the development of selective inhibitors of IKKe and TBK1 remains. The development of high throughput assays to identify inhibitors of TBK1 and IKKe was hindered until recently by the absence of information regarding the substrate specificities of these enzymes. Peptide substrates for IKKe and TBK1 are frequently based on the IKKb phosphorylation sites in IkBa, even though there is no evidence that all IKK family members phosphorylate the same substrate repertoires. In fact, the recently published phosphorylation motifs for IKKa, IKKb and IKKe suggest that these kinases do have overlapping.
of novel small molecule inhibitors of PCSK9. The IKK family of kinases consists of four family members, the canonical IKKa and IKKb, as well as two noncanonical family members, IKKe and TBK1. Together, this family of kinases regulates a myriad of critical cellular processes including inflammation, survival, proliferation, senescence, and autophagy. Consistent with these numerous functions, aberrant IKK signaling can result in susceptibility to diseases such as inflammatory disorders and cancer. The canonical IKK complex, which consists of IKKa, IKKb, and a regulatory subunit, NEMO, is a point of convergence for a variety of stimuli. Upon activation, the canonical IKKs, primarily IKKb, phosphorylate IkBa, the inhibitor of NF-kB, which promotes the ubiquitination and degradation of IkBa. The transcription factor NF-kB is then freed to accumulate in the FTY720 nucleus and U0126 109511-58-2 activate the transcription of a number of target genes involved in inflammatory and stress responses. In contrast to the canonical IKKs, IKKe and TBK1 are activated by a smaller subset of inflammatory stimuli, and are especially critical for antiviral responses. These kinases phosphorylate and activate the transcription factors IRF3, IRF7, and STAT1, promoting a Type 1 interferon response. These kinases also activate NF-kB, but the mechanism by which this occurs in unclear since they do not phosphorylate both of the serines on IkBa which are required for IkBa degradation. IKKe and TBK1 can also promote oncogenesis. For example, IKKe is overexpressed in some breast and ovarian cancers, and TBK1 was recently shown to be important for Ras-induced cell transformation. In spite of the important role these kinases play in both inflammatory and oncogenic signaling, few inhibitors have been identified. BX-795, a small molecule inhibitor of 3phosphoinositide-dependent protein kinase 1, inhibits both IKKe and TBK1 at low nanomolar concentrations in vitro. However, BX795 lacks selectivity as 16 out of 76 tested kinases were inhibited by BX-795 in the nM range. It was also recently shown that a series of azabenzimidazole derivatives inhibits these kinases in the low nM range, but 6 of 79 kinases tested using one of these compounds were inhibited in a range within 10-fold of TBK. These results suggest that IKKe and TBK1 are suitable targets for small molecule inhibitor development, but the need for the development of selective inhibitors of IKKe and TBK1 remains. The development of high throughput assays to identify inhibitors of TBK1 and IKKe was hindered until recently by the absence of information regarding the substrate specificities of these enzymes. Peptide substrates for IKKe and TBK1 are frequently based on the IKKb phosphorylation sites in IkBa, even though there is no evidence that all IKK family members phosphorylate the same substrate repertoires. In fact, the recently published phosphorylation motifs for IKKa, IKKb and IKKe suggest that these kinases do have overlapping.
The feedback was activated in response to doxorubicin and to a lesser extent to the combination doxorubicin/everolimus
In this work, we demonstrate the therapeutic role of mTOR inhibition in chondrosarcoma in localized and advanced phase. Everolimus was tested in an orthotopic rat grade II chondrosarcoma model in macroscopic and “adjuvant” phase both reaching the same conclusion. As a single agent, the mTOR inhibitor everolimus did not cause tumor regression but induced a significant inhibition of tumor growth. Both the size and tumor growth rate were smaller in the everolimus treated groups than in other groups, as observed in other tumor models. Doxorubicin was inactive as single agent; when combined with everolimus, an antagonistic effect was actually observed in the combination group compared to the everolimus treated group. When compared to doxorubicin alone, the combination treatment showed however an increased therapeutic efficiency. Although these data are strongly contrasting with those observed in breast cancer models with paclitaxel and prostate cancer with doxorubicin, a similar effect was recently reported. In human cervical carcinoma xenograft models the addition of everolimus to doxorubicin 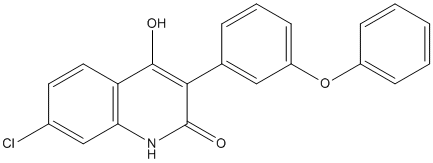 showed an antitumor effect that was not significantly different from doxorubicin monotherapy. The mechanisms underlying this lack of synergism between the two drugs are unclear. One of the side effects of doxorubicin treatment is the induction of reactive oxygen species which in turn can activate the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways. This activation of the mTOR/Akt pathway induced by doxorubicin is reflected by slight increase in Akt phosphorylation in the doxorubicin treated group of our study. In the case of combined treatment this doxorubicin-induced Akt phosphorylation may not be overcome by everolimus at the concentration used and may counteract the antitumor activity of everolimus, as suggested by the higher expression of phospho Akt of the combination group compared to the everolimus-treated one. In the chondrosarcoma model the activity of the mTOR pathway in response to the different treatments was monitored by following activation levels of 4EBP1, S6K as potential surrogate markers of tumor response. Measurement of the phosphorylation status of ph-p70S6K1 and ph-4EBP1 in the tumor itself, confirmed that everolimus resulted in a downregulation of mTOR downstream effectors, whereas doxorubicin had no effect on its phosphorylation status. Everolimus exposure alone did not result in the activation of Akt, a phenomenon already ICI 182780 129453-61-8 reported in other studies. It is known that mTOR inhibitor- can induce a feedback activation of Akt thus contributing to a lesser therapeutic efficiency. This was not observed here with everolimus alone. The data obtained in these experiments indicate that everolimus may affect cell proliferation and metabolism as shown by the down regulation of Ki67 and Glut1 immunostaining. Such an antiproliferative effect has already been reported. The significantly decreased GLUT1 expression observed in the everolimus treated groups appears to be the result of mTOR inhibition and is a consequence of the cross-talk of mTOR downstream effectors with metabolic and hypoxic pathways. Inhibition of mTOR signaling may have direct effect on cell proliferation and also an indirect inhibitor effect on glucose metabolism through the inhibition of HIF1a which expression is (+)-JQ1 1268524-70-4 dependent upon mTOR. The decrease in HIF1a expression seen by immunofluorescence and in the levels of HIF1 a transcript seen by RT-qPCR in tumors of the everolimus treated groups support this bifunctional action of everolimus. Importantly, the present study also investigated the effects of everolimus on residual disease after intralesional curettage in the rat model of chondrosarcoma.
showed an antitumor effect that was not significantly different from doxorubicin monotherapy. The mechanisms underlying this lack of synergism between the two drugs are unclear. One of the side effects of doxorubicin treatment is the induction of reactive oxygen species which in turn can activate the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways. This activation of the mTOR/Akt pathway induced by doxorubicin is reflected by slight increase in Akt phosphorylation in the doxorubicin treated group of our study. In the case of combined treatment this doxorubicin-induced Akt phosphorylation may not be overcome by everolimus at the concentration used and may counteract the antitumor activity of everolimus, as suggested by the higher expression of phospho Akt of the combination group compared to the everolimus-treated one. In the chondrosarcoma model the activity of the mTOR pathway in response to the different treatments was monitored by following activation levels of 4EBP1, S6K as potential surrogate markers of tumor response. Measurement of the phosphorylation status of ph-p70S6K1 and ph-4EBP1 in the tumor itself, confirmed that everolimus resulted in a downregulation of mTOR downstream effectors, whereas doxorubicin had no effect on its phosphorylation status. Everolimus exposure alone did not result in the activation of Akt, a phenomenon already ICI 182780 129453-61-8 reported in other studies. It is known that mTOR inhibitor- can induce a feedback activation of Akt thus contributing to a lesser therapeutic efficiency. This was not observed here with everolimus alone. The data obtained in these experiments indicate that everolimus may affect cell proliferation and metabolism as shown by the down regulation of Ki67 and Glut1 immunostaining. Such an antiproliferative effect has already been reported. The significantly decreased GLUT1 expression observed in the everolimus treated groups appears to be the result of mTOR inhibition and is a consequence of the cross-talk of mTOR downstream effectors with metabolic and hypoxic pathways. Inhibition of mTOR signaling may have direct effect on cell proliferation and also an indirect inhibitor effect on glucose metabolism through the inhibition of HIF1a which expression is (+)-JQ1 1268524-70-4 dependent upon mTOR. The decrease in HIF1a expression seen by immunofluorescence and in the levels of HIF1 a transcript seen by RT-qPCR in tumors of the everolimus treated groups support this bifunctional action of everolimus. Importantly, the present study also investigated the effects of everolimus on residual disease after intralesional curettage in the rat model of chondrosarcoma.
Cetylated form of p53 was found to be localized predominantly in mitochondria where it may play a transcriptionindependent proapoptotic activity
Our previous studies support a protective role of the transcriptional activity of p53 in response to mitotic spindle damage. Down-regulation of p53 could result in a sensitization to PTX as a consequence of prevention of p21WAF1/Cip1 induction in response to PTX. Indeed, we have found that ovarian carcinoma cells selected for resistance to cisplatin and characterized by mutational inactivation of p53 are hypersensitive to PTX. The results presented in this study indicated that ST2782 prevented the upregulation of p21WAF1/Cip1 induced by both PTX, a microtubule polymerising agent and vinorelbine, a microtubule depolymerising agent. The modulation of p21WAF1/Cip1 expression in PTX-treated cells by ST2782 is reminiscent of the effect of pifithrin-a,a transcriptional inhibitor of p53. Relevant to this point is the observation that, in contrast to SAHA, ST2782 and ST3595 induced a dose-dependent down-regulation of p53. The mechanism of this effect is not clearly understood, but likely it is related to modulation of acetylation status of Hsp90, which, as is a protein substrate for the cytoplasmic HDAC6 isoenzyme, may be involved in p53 stabilization. The sensitization of wild-type p53 cells in vitro to PTX by ST3595 was confirmed in tumor xenograft models. The enhancement of the PTX antitumor efficacy by ST3595 was impressive in the osteosarcoma model resulting in complete tumor regression in all treated animals, without evidence of disease at the end of the experiment. These preclinical findings may have therapeutic implications also considering the use of BAY-60-7550 molecular weight nontoxic doses of PTX and the good tolerability of ST3595 following protracted oral administration. In conclusion, given the current interest for combination therapy with HDACi, the present study provides a basis for a rational strategy to improve the taxane-based antitumor therapy. Protein C inhibitor is a 57 kD glycoprotein that belongs to the serine protease inhibitor superfamily of proteins, and exists in many tissues and fluids in humans, including reproductive organs, semen, blood, urine, breast milk and skin. PCI found in blood originates from the liver and is capable of inhibiting several serine proteases involved in the regulation of coagulation and fibrinolysis, including activated protein C, thrombin, factor Xa, various kallikreins and plasminogen activators. Additionally, PCI has been found to have antimicrobial and antitumor properties and thus appears to be a medically interesting versatile protein. PCI has been identified both in the human male and female reproductive tracts. The concentration of PCI in follicular fluid is similar to that in plasma. In contrast, a 40-fold higher concentration is present in the Adriamycin Seminal plasma. Seminal plasma PCI is mainly synthesized in seminal vesicles, where it undergoes glycosylation and is subsequently secreted in an active form. After ejaculation, it is inactivated by forming complexes with prostate-specific antigen, t-PA, u-PA, and tissue kallikrein. Although the function of PCI in seminal plasma is not yet completely understood, evidence showing that PCI plays a 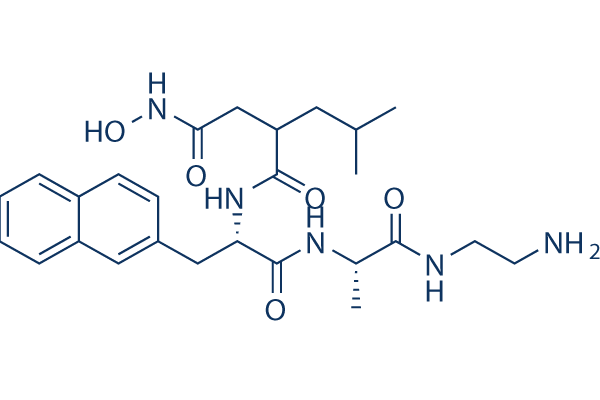 significant role in male fertility has been published. PCI knock-out mice appear to be healthy but males of this genotype are infertile due to abnormal spermatogenesis as the Sertoli cell barrier is destroyed. In a clinical investigation, the inhibitory activities of PCI towards u-PA and tPA were absent in two infertile patients, suggesting that formation of PCI complexes with u-PA and t-PA plays a role in fertilization in the human. Given that the physiological role of PSA is the degradation of the major proteins of seminal coagula, Semenogelin-I and Sg-II, PCI also appears to be involved in the regulation of semen.
significant role in male fertility has been published. PCI knock-out mice appear to be healthy but males of this genotype are infertile due to abnormal spermatogenesis as the Sertoli cell barrier is destroyed. In a clinical investigation, the inhibitory activities of PCI towards u-PA and tPA were absent in two infertile patients, suggesting that formation of PCI complexes with u-PA and t-PA plays a role in fertilization in the human. Given that the physiological role of PSA is the degradation of the major proteins of seminal coagula, Semenogelin-I and Sg-II, PCI also appears to be involved in the regulation of semen.
A critical component of blood clots and an important line of defense against bacterial pathogens
Mutations of p53 and BRAF also seem to not be correlated with sensitivity of BEZ235, because both sensitive and less sensitive cell lines harbor the mutations. Exploration of genetic changes of mTOR, the major target of BEZ235, might someday provide information that may predict therapeutic efficacy. Inhibition of mTORC1 may activate the MAPK pathway through a PI3K-dependent feedback loop, which explains why BEZ235 was seen here to activate ERK1/2 in thyroid cancer cells. Combining BEZ235 with an inhibitor targeting MAPK pathway may be a potential approach to enhance therapeutic efficacy. Recently, the combination of a pan-RAF inhibitor and BEZ235 was shown to induce cell cycle arrest at G0/G1 phase, and had beneficial combination effects in treating thyroid cancer. Similarly, in combination of mTOR and MEK inhibitors also demonstrated therapeutic advantage in treating thyroid cancer, providing further evidence that targeting both PI3K/ mTOR and MAPK pathways is a potential therapeutic strategy for this disease. Approximately 30% of humans are Staphylococcus aureus carriers without symptoms. S. aureus is also one of the most common pathogens in biofilm related infections of indwelling medical devices which are responsible for billions in healthcare cost each year in the United States. Bacteria can attach to the surface of biomaterials or tissues and form a multilayered structure consisting of bacterial cells enclosed in an extracellular polymeric matrix. Bacteria in biofilm are particularly resistant to antibiotic treatment. In addition to the difficulty of effectively inhibiting biofilm with conventional antibiotic therapy, treatment is further complicated by the rise of antibiotic resistance among BYL719 PI3K inhibitor staphylococci. In recent years, methicillin resistance in S. aureus is approaching an epidemic level. The emergence of antibiotic resistance poses an urgent medical problem worldwide. Current antibiotics target a small set of proteins essential for bacterial survival. As a result, antibiotic resistant strains are subjected to a strong positive selection pressure. Inappropriate and excessive use of antibiotics have contributed to the emergence of pathogens that are highly resistant to most currently available antibiotics. The novel approach of inhibiting pathogen virulence while minimizing the selection pressure for resistance holds great promise as an alternative to 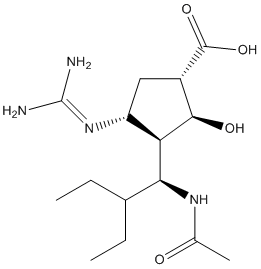 traditional antibiotic treatment. The feasibility of such an approach was demonstrated for Vibrio cholerae infections when a novel small molecule was identified that prevented the production of two critical virulence factors, cholera toxin and the toxin coregulated pilus. Administration of this compound in vivo protected infant mice from V. cholerae. In a similar proof-ofconcept study, a small molecule inhibitor of the membraneembedded sensor histidine kinase QseC was identified. The inhibitor exhibited in vivo protection of mice against infection by Salmonella typhimurium and Francisella tularensis. In a POC study following the same paradigm, we have identified a chemical series of small molecules from a high throughput screen that can inhibit expression of the streptokinase gene in group A streptococcus. We previously demonstrated that SK is a key virulence factor for GAS infection. SK activates human plasminogen into an active serine protease that degrades Evofosfamide fibrin.
traditional antibiotic treatment. The feasibility of such an approach was demonstrated for Vibrio cholerae infections when a novel small molecule was identified that prevented the production of two critical virulence factors, cholera toxin and the toxin coregulated pilus. Administration of this compound in vivo protected infant mice from V. cholerae. In a similar proof-ofconcept study, a small molecule inhibitor of the membraneembedded sensor histidine kinase QseC was identified. The inhibitor exhibited in vivo protection of mice against infection by Salmonella typhimurium and Francisella tularensis. In a POC study following the same paradigm, we have identified a chemical series of small molecules from a high throughput screen that can inhibit expression of the streptokinase gene in group A streptococcus. We previously demonstrated that SK is a key virulence factor for GAS infection. SK activates human plasminogen into an active serine protease that degrades Evofosfamide fibrin.